Broadening the reaction scope of unprotected aldoses via their corresponding nitrones: 1,3-dipolar cycloadditions with alkenes†
Abstract
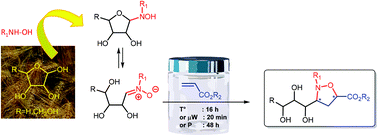
Condensation reactions of unprotected tetroses and pentoses with hydroxylamines afforded nitrones, which were easily converted to densely functionalized isoxazolidines in the presence of electron-poor alkenes. The 1,3-dipolar cycloaddition occurred with good facial discrimination of the chiral nitrone but under rather low endo/exo control. Stereochemistry of isomers was ascertained by chemical correlation with known derivatives from the literature. Microwave activation appeared as the most efficient reaction mode, affording the expected adducts within several minutes whereas hours were needed under standard heating. Alternatively, the transformation proved also possible under high pressure conditions by using a hand pump system, avoiding any energy source. Although water could not be used as the solvent, leading to hydrolysis of the nitrone substrate, a large variety of organic solvents proved efficient. The method has potential use in the preparation of non-ionic carbohydrate-based amphiphiles.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...