Synthesis of gem-difluoroalkenes via nickel-catalyzed allylic defluorinative reductive cross-coupling of trifluoromethyl alkenes with epoxides†
Abstract
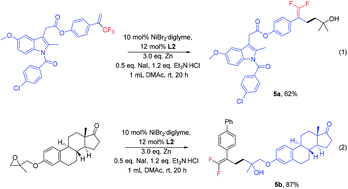
A nickel-catalyzed defluorinative reductive cross-coupling of trifluoromethyl alkenes with epoxides has been developed. Various substituted trifluoromethyl alkenes and epoxides were found to be suitable reaction substrates. This reaction enabled C(sp3)–C(sp3) bond construction through allylic defluorinative cross-coupling of trifluoromethyl alkenes under mild reaction conditions. This methodology was highly compatible with various sensitive functional groups, providing access to a diverse array of functionalized gem-difluoroalkene-containing alcohol compounds.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...