Synthesis of unsymmetrical benzils via palladium-catalysed α-arylation–oxidation of 2-hydroxyacetophenones with aryl bromides†
Abstract
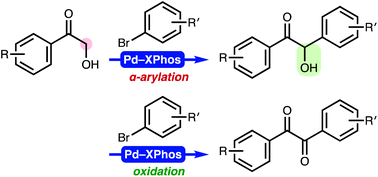
A diverse set of unsymmetrically substituted benzils were facilely synthesised by a cross-coupling reaction between 2-hydroxyacetophenones and aryl bromides in the presence of a palladium catalyst. Experimental studies suggested a reaction mechanism involving a one-pot tandem palladium-catalysed α-arylation and oxidation, where aryl bromides play a dual role as mild oxidants as well as arylating agents.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...