S-Glycosides: synthesis of S-linked arabinoxylan oligosaccharides†
Abstract
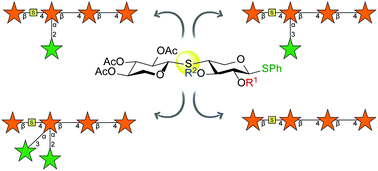
S-Glycosides are important tools for the elucidation of specific protein–carbohydrate interactions and can significantly aid structural and functional studies of carbohydrate-active enzymes, as they are often inert or act as enzyme inhibitors. In this context, this work focuses on the introduction of an S-linkage into arabinoxylan oligosaccharides (AXs) in order to obtain a small collection of synthetic tools for the study of AXs degrading enzymes. The key step for the introduction of the S-glycosidic linkage involved anomeric thiol S-alkylation of an orthogonally protected L-arabinopyranoside triflate. The resulting S-linked disaccharide was subsequently employed in a series of glycosylation reactions to obtain a selectively protected tetrasaccharide. This could be further elaborated through chemoselective deprotection and glycosylation reactions to introduce branching L-arabinofuranosides.
- This article is part of the themed collections: Chemical Biology in OBC and Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...