An alternative approach to the synthesis of the three fragments of anachelin H†
Abstract
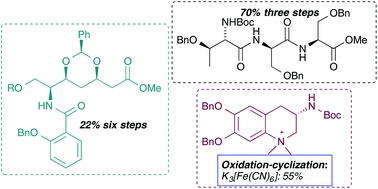
The synthesis of the fully protected peptide, polyketide and alkaloid fragments of anachelin H is presented. The peptide fragment was prepared using a liquid phase peptide synthesis; the polyketide fragment was synthetized using a cross metathesis and an intramolecular oxa-Michael reaction as the key steps to introduce the desired stereochemistry; finally, the alkaloid fragment was obtained by an oxidative cyclization of a catechol derivative using potassium ferricyanide. The synthesis of all fragments was based on the use of natural amino acids as sources of asymmetry. The independent synthesis of the three fragments should allow more efficient biological studies on the fragments instead of the whole natural product. Experiments to illustrate the coupling of fragments and the effectiveness of the convergent strategy are also described.
- This article is part of the themed collections: Celebrating Latin American Talent in Chemistry and Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...