Merging singlet-oxygen induced furan oxidations with organocatalysis: synthesis of enantiopure cyclopentanones and hydrindanes†
Abstract
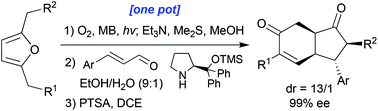
A new methodology is described herein which converts simple and readily accesible furan substrates into complex enantio-enriched carbocyclic skeletons through the implementation of a simple one-pot procedure. Singlet oxygen furan photoxygenation affords an enedione which then participates in an organocatalysed double-Michael reaction with an enal to furnish a cyclopentanone structure with up to four new contiguous stereogenic centres. The enantioselectivity and diastereoselectivity of this process are both excellent. If desired, further aldol-annulation steps can be appended to the cascade reaction sequence to afford key enantiopure hydrindane motifs.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...