Asymmetric total syntheses of naturally occurring α,β-enone-containing RALs, L-783290 and L-783277 through intramolecular base-mediated macrolactonization reaction†
Abstract
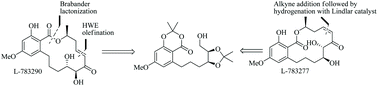
Asymmetric total synthesis of two naturally occurring α,β-enone containing RALs, L-783290 and L-783277 is described in this article. An E-selective Horner–Wadsworth–Emmons (HWE) olefination was used as a key reaction to construct the C7′–C8′ olefinic unsaturation in L-783290. An enantiopure alkyne addition to the aldehyde followed by Z-selective partial reduction was employed to construct the C7′–C8′ olefinic unsaturation in L-783277. Biomimetic lactonization reaction was used to construct the macrolactone core in both the target molecules.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...