Chemoselective photocatalytic oxidation of alcohols to aldehydes and ketones by nitromethane on titanium dioxide under violet 400 nm LED light irradiation†
Abstract
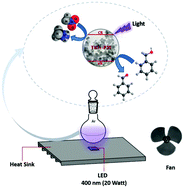
In this study, for the first time, nitroalkanes, especially nitromethane, have been used as electron acceptors for the highly chemoselective oxidation of alcohols in the presence of a TiO2 photocatalyst under 400 nm LED irradiation. The reactions showed excellent selectivity for the production of aldehydes. Interestingly, aldehydes such as benzaldehyde and p-methoxybenzaldehyde are stable under the reaction conditions. In the case of the use of 2-nitropropane and 2-methyl-2-nitropropane, the product imine, which is the result of the reaction of the aldehyde with aliphatic amine, is also obtained.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...