Examination of pinanediol–boronic acid ester formation in aqueous media: relevance to the relative stability of trigonal and tetrahedral boronate esters†
Abstract
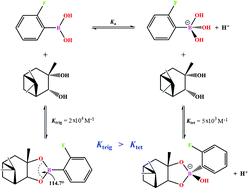
The interaction of pinanediol with 2-fluorophenylboronic acid and several other substituted phenylboronic acids was studied in 40% vol. aqueous acetonitrile by 1H and 11B NMR, potentiometric and spectrophotometric titrations at variable pH values. The experimental results reveal the formation of a very stable trigonal ester (Ktrig ≈ 2 × 104 M−1) and a significantly less stable tetrahedral hydroxocomplex (Ktet ≈ 5 × 103 M−1) in contrast to the traditionally observed inverted order of stabilities Ktrig < Ktet. Comparison of the crystal structure of the trigonal ester isolated from aqueous acetonitrile with the DFT simulated structure of the respective hydroxocomplex shows that an unusual order of stabilities Ktrig > Ktet is observed in spite of the existence of the usual strain release effect in the O–B–O angle considered responsible for the typically observed increased stability of the tetrahedral hydroxocomplex. A complementary study of the stability of the six–membered cyclic boronate esters of chromotropic acid demonstrated the order Ktrig ≪ Ktet although the strain was absent in these esters. The results for m-, p-substituted phenylboronic acids show that the stability of both five- and six-membered trigonal esters formed with pinanediol and chromotropic acid, respectively, is insensitive to electronic effects but the electron accepting substituents stabilize the hydroxocomplexes. It follows from the whole set of results that Ktet can be much larger than Ktrig in the absence of the strain, but with a sufficiently acidic diol, and that the presence of the strain does not necessarily make Ktet larger than Ktrig for a less acidic diol with a purely saturated hydrocarbon backbone. Thus, the electronic effects manifested in the acidity of the diol appear to be more significant than the strain release effect in determining the Ktet/Ktrig ratio.
- This article is part of the themed collections: Celebrating Latin American Talent in Chemistry and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...