Carbonylative Suzuki–Miyaura couplings of sterically hindered aryl halides: synthesis of 2-aroylbenzoate derivatives†
Abstract
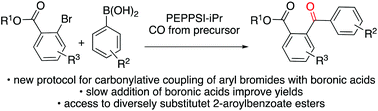
We have developed a carbonylative approach to the synthesis of diversely substituted 2-aroylbenzoate esters featuring a new protocol for the carbonylative coupling of aryl bromides with boronic acids and a new strategy to favour carbonylative over non-carbonylative reactions. Two different synthetic pathways – (i) the alkoxycarbonylation of 2-bromo benzophenones and (ii) the carbonylative Suzuki–Miyaura coupling of 2-bromobenzoate esters – were evaluated. The latter approach provided a broader substrate tolerance, and thus was the preferred pathway. We observed that 2-substituted aryl bromides were challenging substrates for carbonylative chemistry favouring the non-carbonylative pathway. However, we found that carbonylative Suzuki–Miyaura couplings can be improved by slow addition of the boronic acid, suppressing the unwanted direct Suzuki coupling and, thus increasing the yield of the carbonylative reaction.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...