Ruthenium-catalysed cyclisation reactions of 1,11-dien-6-ynes leading to biindenes†
Abstract
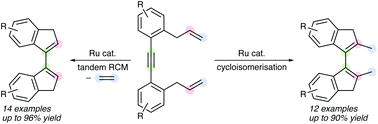
1,2-Bis(2-allylphenyl)ethynes undergo cycloisomerisation reactions in the presence of Cp*Ru(II) catalysts to produce 2,2′-dimethyl-3H,3′H-1,1′-biindenes. On the other hand, tandem ring-closing metathesis of 1,2-bis(2-allylphenyl)ethynes using the Hoveyda–Grubbs 2nd generation catalyst led to the formation of 2,2′-unsubstituted biindenes. Various symmetrical and unsymmetrical bicyclic dienes were prepared by these ruthenium-based cyclisation methods.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...