Sequential conjugation methods based on triazole formation and related reactions using azides
Abstract
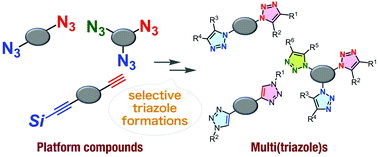
The recent remarkable progress in azide chemistry has realized sequential conjugation methods with selective 1,2,3-triazole formation. On the basis of the diverse reactivities of azides and azidophiles, including terminal alkynes and cyclooctynes, various selective reactions to furnish triazoles and a wide range of platform molecules, such as diynes, diazides, triynes, and triazides, have been developed so far for bis- and tris(triazole) syntheses. This review highlights recent transformations involving selective triazole formation, allowing the efficient preparation of unsymmetric bis- and tris(triazole)s using diverse platform molecules.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...