γ-C (sp3)–H bond functionalisation of α,β-unsaturated amides through an umpolung strategy†
Abstract
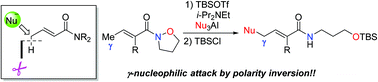
The nucleophilic γ-phenylation and γ-alkylation of α,β-unsaturated amides have been developed. This umpolung reaction allows the regioselective introduction of phenyl and alkyl groups to a vinylketene N,O-acetal, which is generated in situ from an α,β-unsaturated N-alkoxyamide, followed by N–O bond cleavage in a two-step, one-pot process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...