Iodine(iii) promoted ring-rearrangement reaction of 1-arylamino-2-oxocyclopentane-1-carbonitriles to synthesize N-aryl-δ-valerolactams†
Abstract
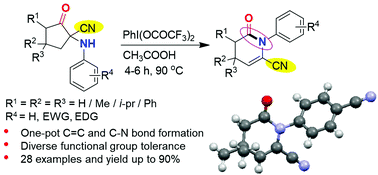
The first example of N-aryl-δ-valerolactam synthesis via an intramolecular ring rearrangement reaction of 1-arylamino-2-oxocyclopentane-1-carbonitrile promoted by phenyliodine bis(trifluoroacetate) (PIFA) was reported. We show that this unprecedented regio-selective ring-rearrangement reaction driven by hypervalent iodine (PIFA) involves C5–H elimination, C1–C2 bond opening, and C1–N bond rearrangement steps and restraining the leaving tendency of the CN group. The structure of the lactam was further confirmed by single crystal X-ray diffraction (XRD) analysis. The present protocol showed a diverse array of functional group tolerance under the reaction conditions and offered good to excellent yields of lactams.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...