Iron-catalyzed regioselective alkylation of 1,4-quinones and coumarins with functionalized alkyl bromides†
Abstract
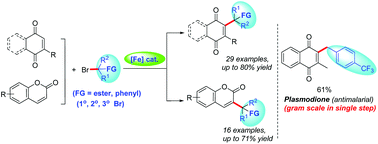
A simple and efficient Fe-catalyzed regioselective alkylation of 1,4-quinones and coumarins, using functionalized alkyl bromides as alkylating reagents, has been developed. The reaction proceeds under mild conditions with the addition of alkyl bromides to a wide range of 1,4-quinone and coumarin derivatives with a broad substrate scope and wide functional group tolerance to provide the products in good yields. Further application of these strategies could be extended to important biologically active antimalarial lead drugs, such as plasmodione on a gram scale in a single step for medicinal purposes.

 Please wait while we load your content...
Please wait while we load your content...