6-Deoxy-6-fluoro galactofuranosides: regioselective glycosylation, unexpected reactivity, and anti-leishmanial activity†
Abstract
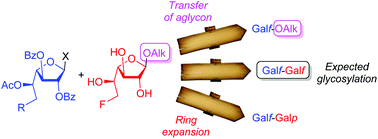
Selective glycosylation of the C-6 fluorinated galactofuranosyl acceptor 2 was studied with four galactofuranosyl donors. It was highlighted that this electron-withdrawing atom strongly impacted the behavior of the acceptor, thus leading to unprecedented glycosylation pathways. Competition between expected glycosylation of 2, ring expansion of this acceptor and furanosylation, and intermolecular aglycon transfer was observed. Further investigation of the fluorinated synthetic compounds showed that the presence of fluorine atom contributed to increase the inhibition of the growth of Leishmania tarentolae, a non-pathogenic strain of Leishmania.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...