One-pot synthesis of 2-azolylimidazole derivatives through a domino addition/A3 coupling/cyclization process under copper catalysis†
Abstract
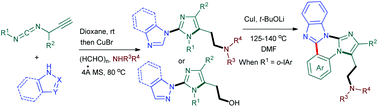
A novel one-pot approach for the synthesis of multi-substituted 2-imidazolylimidazoles, 2-pyrazolylimidazoles and 2-indazolylimidazoles was developed through a domino addition/A3 coupling/cyclization process under copper catalysis. A variety of aminoethyl- or hydroxylethyl-tethered 2-azolylimidazole derivatives were conveniently and efficiently assembled in one pot using N-propargylcarbodiimides, azoles, paraformaldehyde and secondary amines as starting materials. The products containing an o-iodoaryl group could be further converted to imidazo[1,2-c]imidazo[1,2-a]quinazoline derivatives through a copper-catalyzed intramolecular C–H arylation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...