The contribution of achiral residues in the laspartomycin family of calcium-dependent lipopeptide antibiotics†
Abstract
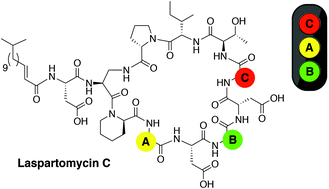
The growing threat of antibacterial resistance is a global concern. The so-called calcium-dependent lipopeptide antibiotics (CDAs) have emerged as a promising source of new antibiotic agents that are rich in structural and mechanistic diversity. Over forty unique CDAs have been identified to date and share a number of common features. Recent efforts in our group have provided new mechanistic and structural insights into the laspartomycin family of CDAs. We here describe investigations aimed at probing the role of the three glycine residues found in the laspartomycin peptide macrocycle. In doing so laspartomycin analogues containing the achiral 2-aminoisobutyric acid (AIB) as well as L- or D-alanine in place of glycine were prepared and their antibacterial activities evaluated.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...