Stereochemistry, lipid length and branching influences Mincle agonist activity of monoacylglycerides†
Abstract
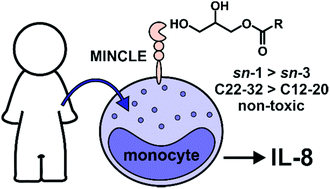
Herein, we report on the synthesis of a series of enantiomerically pure linear, iso-branched, and α-branched monoacyl glycerides (MAGs) in 63–72% overall yield. The ability of the MAGs to signal through human macrophage inducible C-type lectin (hMincle) using NFAT-GFP reporter cells was explored, as was the ability of the compounds to activate human monocytes. From these studies, MAGs with an acyl chain length ≥C22 were required for Mincle activation and the production of interleukin-8 (IL-8) by human monocytes. Moreover, the iso-branched MAGs led to a more pronounced immune response compared to linear MAGs, while an α-branched MAG containing a C-32 acyl chain activated cells to a higher degree than trehalose dibehenate (TDB), the prototypical Mincle agonist. Across the compound classes, the activity of the sn-1 substituted isomers was greater than the sn-3 counterparts. None of the representative compounds were cytotoxic, thus mitigating cytotoxicity as a potential mediator of cellular activity. Taken together, 6h (sn-1, iC26+1), 8a (sn-1, C32) and 8b (sn-3, C32) exhibited the best immunostimulatory properties and thus, have potential as vaccine adjuvants.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...