Hypersensitive azobenzenes: facile synthesis of clickable and cleavable azo linkers with tunable and high reducibility†
Abstract
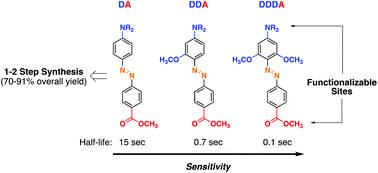
The aim of this work is to show that by increasing the number of donor substituents in a donor/acceptor system, the sensitivity of the azobenzene linkage towards a reductive cleavage reaction can be enhanced to unprecedented high levels. For instance, in a triple-donor system, less than a second constitutes the half-life of the azo (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) bond. Synthetic access to such redox active scaffolds is highly practical and requires only 1–2 synthetic steps. The fundamental molecular design is also adaptable. This is demonstrated through scaffold functionalization by azide, tetraethylene glycol, and biotin groups. The availability of the azide group is shown in a copper-free ‘click’ reaction suitable in context with protein conjugation and proteomics application. Finally, the clean nature of the scission process is demonstrated with the help of liquid chromatography coupled with mass analysis. This work, therefore, describes development of cleavable azobenzene linkers that can be accessed with synthetic ease, can be multiply functionalized, and show a clean and rapid response to mild reducing conditions.
N) bond. Synthetic access to such redox active scaffolds is highly practical and requires only 1–2 synthetic steps. The fundamental molecular design is also adaptable. This is demonstrated through scaffold functionalization by azide, tetraethylene glycol, and biotin groups. The availability of the azide group is shown in a copper-free ‘click’ reaction suitable in context with protein conjugation and proteomics application. Finally, the clean nature of the scission process is demonstrated with the help of liquid chromatography coupled with mass analysis. This work, therefore, describes development of cleavable azobenzene linkers that can be accessed with synthetic ease, can be multiply functionalized, and show a clean and rapid response to mild reducing conditions.


 Please wait while we load your content...
Please wait while we load your content...