Conformation modification of terthiophene during the on-surface synthesis of pure polythiophene†
Abstract
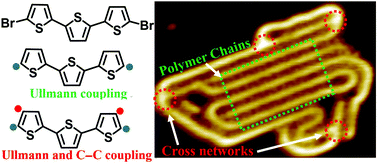
On-surface coupling under ultra-high vacuum is employed as a versatile approach to synthesize pure polythiophene from a 5,5′′-dibromo-2,2′:5′,2′′-terthiophene (DBTT) precursor and the corresponding temperature-dependent stepwise reaction mechanism is systematically studied by scanning tunneling microscopy (STM). After thermal deposition of the precursor onto a Au(111) surface that is kept at room temperature, a triangle-like pattern and a linear self-assembled pattern are formed with different molecular coverages through Br⋯Br⋯S halogen bonds and Br⋯Br type-I contact bonds, respectively. In the self-assembled nanostructures, the thiophene units adopt trans-conformation. Mild annealing promotes the structural transition of both nanostructures into ordered zigzag organometallic linear chains with all-cis configured thiophene units connected through coordination bonds to the Au adatoms. Such conformational variety is easily recognized by STM, particularly in the case of DBTT-CH3 with the extra –CH3 signals. The covalently coupled products from the DBTT precursor are obtained by further annealing the organometallic intermediate at higher temperatures, which leads to the removal of Au atoms and the formation of ordered polymer chains and disordered polythiophene networks. Further characterization suggests that the reaction mechanism is associated with Ullmann-type coupling to form the ordered chains as well as Ullmann-type and dehydrogenative C–C coupling to fabricate cross-linked polymer networks. Compared with the on-surface synthesis process of DBTT on the Cu(111) surface, it can be confirmed that the Au adatoms are vital to synthesize polythiophene. These findings provide important insight into the reaction mechanism of on-surface synthesized pure polythiophene and on-surface coupling can potentially be applied to synthesize other functional conjugated polymers.
- This article is part of the themed collection: 2020 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...