Interfacial water and ion distribution determine ζ potential and binding affinity of nanoparticles to biomolecules†
Abstract
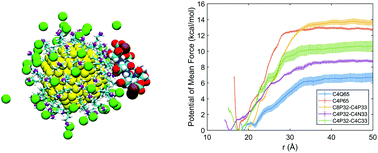
The molecular features that dictate interactions between functionalized nanoparticles and biomolecules are not well understood. This is in part because for highly charged nanoparticles in solution, establishing a clear connection between the molecular features of surface ligands and common experimental observables such as ζ potential requires going beyond the classical models based on continuum and mean field models. Motivated by these considerations, molecular dynamics simulations are used to probe the electrostatic properties of functionalized gold nanoparticles and their interaction with a charged peptide in salt solutions. Counterions are observed to screen the bare ligand charge to a significant degree even at the moderate salt concentration of 50 mM. As a result, the apparent charge density and ζ potential are largely insensitive to the bare ligand charge densities, which fall in the range of ligand densities typically measured experimentally for gold nanoparticles. While this screening effect was predicted by classical models such as the Manning condensation theory, the magnitudes of the apparent surface charge from microscopic simulations and mean-field models are significantly different. Moreover, our simulations found that the chemical features of the surface ligand (e.g., primary vs. quaternary amines, heterogeneous ligand lengths) modulate the interfacial ion and water distributions and therefore the interfacial potential. The importance of interfacial water is further highlighted by the observation that introducing a fraction of hydrophobic ligands enhances the strength of electrostatic binding of the charged peptide. Finally, the simulations highlight that the electric double layer is perturbed upon binding interactions. As a result, it is the bare charge density rather than the apparent charge density or ζ potential that better correlates with binding affinity of the nanoparticle to a charged peptide. Overall, our study highlights the importance of molecular features of the nanoparticle/water interface and underscores a set of design rules for the modulation of electrostatic driven interactions at nano/bio interfaces.


 Please wait while we load your content...
Please wait while we load your content...