Abstract
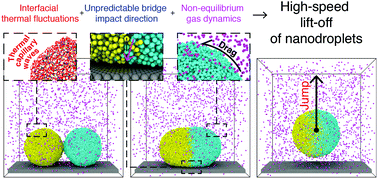
Next-generation processor-chip cooling devices and self-cleaning surfaces can be enhanced by a passive process that requires little to no electrical input, through coalescence-induced nanodroplet jumping. Here, we describe the crucial impact thermal capillary waves and ambient gas rarefaction have on enhancing/limiting the jumping speeds of nanodroplets on low adhesion surfaces. By using high-fidelity non-equilibrium molecular dynamics simulations in conjunction with well-resolved volume-of-fluid continuum calculations, we are able to quantify the different dissipation mechanisms that govern nanodroplet jumping at length scales that are currently difficult to access experimentally. We find that interfacial thermal capillary waves contribute to a large statistical spread of nanodroplet jumping speeds that range from 0–30 m s−1, where the typical jumping speeds of micro/millimeter sized droplets are only up to a few m s−1. As the gas surrounding these liquid droplets is no longer in thermodynamic equilibrium, we also show how the reduced external drag leads to increased jumping speeds. This work demonstrates that, in the viscous-dominated regime, the Ohnesorge number and viscosity ratio between the two phases alone are not sufficient, but that the thermal fluctuation number (Th) and the Knudsen number (Kn) are both needed to recover the relevant molecular physics at nanoscales. Our results and analysis suggest that these dimensionless parameters would be relevant for many other free-surface flow processes and applications that operate at the nanoscale.


 Please wait while we load your content...
Please wait while we load your content...