Selective detection of Fe3+ ions based on fluorescence MXene quantum dots via a mechanism integrating electron transfer and inner filter effect†
Abstract
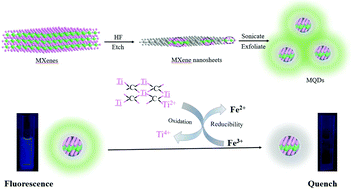
Fluorescence quantum dots (QDs) are promising functional nanomaterials in chemical biology and environmental applications, where an analyte-induced responsive system is beneficial for detecting numerous life-related molecules and pollutants. Here, fluorescent Ti3C2 MXene quantum dots (MQDs) with the size of 1.75 nm were synthesized by a simple method of hydrofluoric acid etching and dimethyl sulfoxide exfoliation to form nanosheets followed by a one-step ultrasound method. The as-synthesized MQDs showed excitation-dependent behaviour along with a fluorescence quantum yield value of 7.7%. In addition, the fluorescence of the MQDs can be significantly suppressed by Fe3+. The mechanism for the fluorescence quenching of the MQDs was systematically investigated, which was attributed to the oxidation–reduction reaction between the MQDs and Fe3+ and the inner filter effect (IFE), different from the reported Förster resonant energy transfer (FRET) mechanism for MXene nanosheets. Based on this trait, a fluorescence method for Fe3+ detection based on MQDs was demonstrated with high sensitivity and selectivity, and the limit of detection was 310 nM. The proposed method was successfully used for the sensitive detection of Fe3+ in serum and sea water. This work will not only help to understand the selectivity mechanisms of MQDs as fluorescent probes for metal ions, but also provide a smart sensing platform in biological and environmental detection.


 Please wait while we load your content...
Please wait while we load your content...