Hollow porous prismatic graphitic carbon nitride with nitrogen vacancies and oxygen doping: a high-performance visible light-driven catalyst for nitrogen fixation†
Abstract
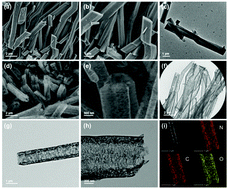
Hollow porous prismatic graphitic carbon nitride with nitrogen vacancies and oxygen doping was successfully constructed using dicyandiamidine as the only raw material via a facile two-step strategy of a low-temperature hydrothermal method followed by a subsequent calcination process. The as-obtained graphitic carbon nitride showed a hollow prismatic morphology with loose spongy-like walls, a hierarchical pore structure, and a specific surface area of 220.16 m2 g−1. Such graphitic carbon nitride exhibited an ultrahigh nitrogen fixation rate of 118.8 mg L−1 h−1 gcat−1 under visible light irradiation and showed excellent stability during the reactions. A possible mechanism for photocatalytic nitrogen fixation on the catalyst was proposed as follows: under visible-light irradiation, graphitic carbon nitride with nitrogen vacancies and oxygen doping underwent charge separation to generate electron–hole pairs, and then the photogenerated electrons on the conduction band were quickly transferred to the nitrogen vacancy induced mid-gap state; consequently, the trapped electrons reacted with the activated nitrogen on the nitrogen vacancies to produce ammonia. The significant enhancement in the photocatalytic nitrogen fixation performance of graphitic carbon nitride can be attributed to its unique hollow prismatic morphology with a loose porous structure, fully exposed active sites of nitrogen vacancies, more negative conduction band, suitable visible-light response and the efficient separation of photogenerated electron–hole pairs.


 Please wait while we load your content...
Please wait while we load your content...