A stable, highly oxidizing radical cation†
Abstract
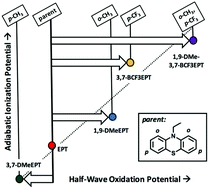
Highly oxidizing radical cation salts can be used as chemical oxidants in a wide variety of applications. While some are commercial and others can be made, stability has been a problem with many of these organic-based reagents. We sought a method to increase redox potentials of organic compounds, to yield radical cation salts that do not suffer the same instability as their triarylamine counterparts. Using phenothiazines, we (i) blocked the positions para to nitrogen with a substituent containing strong covalent bonds, using an electron-withdrawing group to increase oxidation potential, while at the same time (ii) introduced strain at the positions ortho to nitrogen to further raise the oxidation potential by preventing geometric relaxation of the oxidized state. Here we synthesized the phenothiazine derivative, N-ethyl-1,9-dimethyl-3,7-bis(trifluoromethyl)phenothiazine to test this hypothesis. Indeed, oxidation potentials reflect additive substituent effects, yielding a high-potential redox couple with a stable radical cation. Stability tests in solution and the solid state show that the radical cation form of this phenothiazinium is stable and can be used to oxidize other organic compounds in solution.


 Please wait while we load your content...
Please wait while we load your content...