Mixed N-aryl/alkyl substitution favours an unusual tautomer of near-infrared absorbing azacalixphyrins†
Abstract
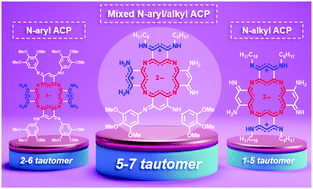
Azacalixphyrins are bis-zwitterionic aromatic macrocycles that feature absorption properties in the near-infrared range. Their N-substitution is an efficient strategy for tuning the absorption maxima by stabilizing different tautomeric forms depending on the nature of the substituent (alkyl or aryl give 1–5 or 2–6 tautomers, respectively). This work depicts the synthesis of a new azacalixphyrin presenting both aryl and alkyl substituents. The joint experimental and theoretical study supports that the substitution pattern can be manipulated to counterbalance the repulsion of the two peripheral cationic charges to favour an unusual 5–7 tautomer.


 Please wait while we load your content...
Please wait while we load your content...