Biocatalytic green alternative to existing hazardous reaction media: synthesis of chalcone and flavone derivatives via the Claisen–Schmidt reaction at room temperature†
Abstract
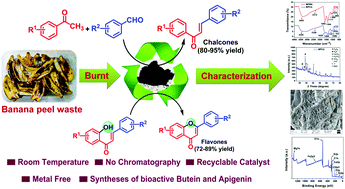
Owing to the increasing amount of waste materials around the globe, the conversion of waste or secondary by-products to value-added products for various applications has gained significant interest. Herein, two novel agro-food waste products, Musa sp. ‘Malbhog’ peel ash (MMPA) and Musa Champa Hort. ex Hook. F. peel ash (MCPA) are used as catalysts to promote an inexpensive, efficient and eco-friendly carbon–carbon bond forming crossed aldol reaction at room temperature in solvent free conditions. Furthermore, the resulting products were subjected to reactions with these promoters in an oxygen atmosphere and led to the formation of novel flavone derivatives. Moreover, the used catalysts were properly characterized using different sophisticated analytical techniques such as Fourier-transform infrared spectroscopy (FT-IR), X-ray diffractometry (XRD), Brunauer–Emmett–Teller analysis (BET), Raman spectroscopy, scanning electron microscopy energy dispersive X-ray spectroscopy (SEM-EDS), transition electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and thermogravimetric analysis (TGA) along with element detection using atomic absorption spectroscopy and ion chromatographic methods. These two approaches are metal free, as well as being devoid of any extra additives, co-catalysts, harsh conditions, the use of column chromatography for purification and result in a higher yield of the product within a short space of time. The catalytic abilities of the promoter were also examined to synthesize important bioactive molecules such as butein and apigenin at room temperature. With gram scale synthesis of the chalcone derivatives, the used catalysts (MMPA and MCPA) were further reused for five cycles and did not demonstrate any loss in catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...