Syntheses, spectral and chiral properties and DNA interactions of multi-heterocyclic di- and trinuclear boron complexes†
Abstract
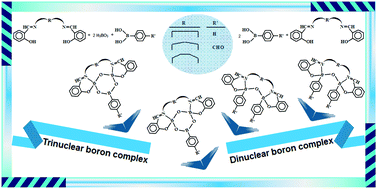
Tetrahedrally coordinated multi-heterocyclic boron complexes are stable, but much less investigated inorganic ring systems. In the present study, a series of dinuclear (2aI–2cI and 2aII–2cII) and trinuclear (3aI–3cI and 3aII–3cII) boron complexes were synthesized from SalenH2 type symmetrical bulky ligands [HOArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–R–N
N–R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHArOH; R = (CH2)n, n = 2 (1a), 3 (1b) and 4 (1c)], arylboronic acids (phenylboronic acid and 4-formylphenylboronic acid), and boric acid for the investigation of their spectral, stereogenic and DNA cleavage activities. The Salen-boron complexes have two equivalent chiral B-centers, giving rise to diastereoisomers. The stereogenic properties of these complexes were investigated by nuclear magnetic resonance (NMR) and circular dichroism (CD) spectroscopies. The stereoisomers were compared with each other for two different architectural types and a total of 12 Salen-boron complexes with seven- to nine-membered [(B–O–B)–(N–R–N)] heterocycles. The combination of NMR and CD spectra of the complexes shows that the boron complexes (2bI and 3bII) and (2bII and 3bI) from the (CH2)3 precursor give only a cis–meso (RS/SR) isomer and only one trans-enantiomer (RR or SS), respectively. The complexes (2cI, 3cI and 3cII) from the (CH2)4 precursor give only one enantiomer (RR or SS), whereas the boron complexes (2aI, 2aII, 3aI and 3aII) from the (CH2)2 precursor and (2cII) from the (CH2)4 precursor form two diastereoisomers as one enantiomer (RR or SS) and one meso (RS/SR). Furthermore, the DNA cleavage activities of the adducts were determined using agarose gel electrophoresis and UV absorption in order to compare the cleavage efficiency of the boron complexes depending on the type of complexes (dinuclear or trinuclear) and the number of members in the heterocyclic frameworks. In this assay, the seven-membered trinuclear boron complex (3bII) showed the highest cleavage efficiency.
CHArOH; R = (CH2)n, n = 2 (1a), 3 (1b) and 4 (1c)], arylboronic acids (phenylboronic acid and 4-formylphenylboronic acid), and boric acid for the investigation of their spectral, stereogenic and DNA cleavage activities. The Salen-boron complexes have two equivalent chiral B-centers, giving rise to diastereoisomers. The stereogenic properties of these complexes were investigated by nuclear magnetic resonance (NMR) and circular dichroism (CD) spectroscopies. The stereoisomers were compared with each other for two different architectural types and a total of 12 Salen-boron complexes with seven- to nine-membered [(B–O–B)–(N–R–N)] heterocycles. The combination of NMR and CD spectra of the complexes shows that the boron complexes (2bI and 3bII) and (2bII and 3bI) from the (CH2)3 precursor give only a cis–meso (RS/SR) isomer and only one trans-enantiomer (RR or SS), respectively. The complexes (2cI, 3cI and 3cII) from the (CH2)4 precursor give only one enantiomer (RR or SS), whereas the boron complexes (2aI, 2aII, 3aI and 3aII) from the (CH2)2 precursor and (2cII) from the (CH2)4 precursor form two diastereoisomers as one enantiomer (RR or SS) and one meso (RS/SR). Furthermore, the DNA cleavage activities of the adducts were determined using agarose gel electrophoresis and UV absorption in order to compare the cleavage efficiency of the boron complexes depending on the type of complexes (dinuclear or trinuclear) and the number of members in the heterocyclic frameworks. In this assay, the seven-membered trinuclear boron complex (3bII) showed the highest cleavage efficiency.


 Please wait while we load your content...
Please wait while we load your content...