Pictet–Spengler condensations using 4-(2-aminoethyl)coumarins†
Abstract
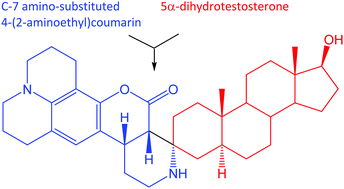
Androgen-deprivation therapy (ADT) is only a palliative measure, and prostate cancer invariably recurs in a lethal, castration-resistant form (CRPC). Prostate cancer resists ADT by metabolizing weak, adrenal androgens to growth-promoting 5α-dihydrotestosterone (DHT), the preferred ligand for the androgen receptor (AR). Developing small-molecule inhibitors for the final steps in androgen metabolic pathways that utilize 17-oxidoreductases required probes that possess fluorescent groups at C-3 and intact, naturally occurring functionality at C-17. Application of the Pictet–Spengler condensation to substituted 4-(2-aminoethyl)coumarins and 5α-androstane-3-ones furnished spirocyclic, fluorescent androgens at the desired C-3 position. Condensations required the presence of activating C-7 amino or N,N-dialkylamino groups in the 4-(2-aminoethyl)coumarin component of these condensation reactions. Successful Pictet–Spengler condensation, for example, of DHT with 9-(2-aminoethyl)-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinolin-11-one led to a spirocyclic androgen, (3R,5S,10S,13S,17S)-17-hydroxy-10,13-dimethyl-1,2,2′,3′,4,5,6,7,8,8′,9,9′,10,11,12,12′,13,13′,14,15,16,17-docosahydro-7′H,11′H-spiro-[cyclopenta[a]phenanthrene-3,4′-pyrido[3,2,1-ij]pyrido[4′,3′:4,5]pyrano[2,3-f]quinolin]-5′(1′H)-one. Computational modeling supported the surrogacy of the C-3 fluorescent DHT analog as a tool to study 17-oxidoreductases for intracrine, androgen metabolism.


 Please wait while we load your content...
Please wait while we load your content...