The synthesis of biphenyl through C–H bond activation in benzene over a Pd catalyst supported on graphene oxide†
Abstract
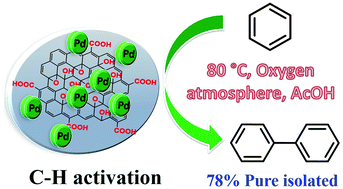
This is the first report on carbon–hydrogen (C–H) bond activation in benzene over a palladium catalyst supported on graphene oxide (GO) leading to the sole formation of biphenyl with a yield of 78%. The reaction was performed for 12 h in the presence of acetic acid and oxygen at 80 °C. XPS studies indicated that in the initial catalyst, Pd is mainly present as Pd(II) species on the GO surface. The interaction of these species with acetic acid during the reaction generates Pd acetate species. Density functional theory (DFT) studies revealed that the adsorption of the first benzene molecule on the Pd acetate is weak (0.15 eV) and the energy barrier of the following C–H bond scission is high, equal to 1.67 eV. The adsorption of the second benzene molecule is relatively strong (0.40 eV); acetic acid molecules are then released, leaving the biphenyl Pd intermediate, which enables biphenyl molecule formation. The presence of oxygen and acetic acid is needed for closing the catalytic cycle via the regeneration of the reactive Pd acetate.


 Please wait while we load your content...
Please wait while we load your content...