A comprehensive study on the impact of chemical structures of ionic liquids on the solubility of ethane†
Abstract
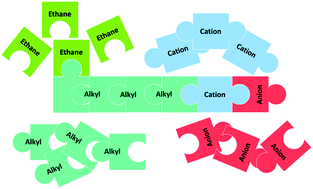
A clear understanding of the solubility of gas in ionic liquids (ILs) is important for assessing the feasibility of this fluid as an alternative solvent in gas capture and separation processes. In this work, the solubility of ethane in 16 commercial ILs was measured and predicted using the conductor-like screening model for real solvents (COSMO-RS) at four different temperatures and at pressures up to 2 MPa. Henry's law constants were calculated from the obtained experimental data. The broad range of the chosen set of ILs permits a systematic exploration of the impacts of cation, anion, and alkyl chain length on the solubility of ethane in these fluids. The results showed that the solubility of ethane in ILs could be increased by increasing pressure and decreasing temperature. The obtained experimental and COSMO-RS results showed that (i) the solubility of ethane could be enhanced by increasing the nonpolar character of ionic liquids, such as the alkyl chain length of both cation and anion, (ii) fluorination of the anion reduced the solubility of ethane due to preferential solvation of the solute in ILs, and (iii) the increase of ethane solubility in the series [CnC1im][Tf2N] is not linear with increasing alkyl chain length, which can be explained based on the nano-structural organization of the ILs. Finally, computational modelling using COSMO-RS showed that the increase in the solubility of ethane in ILs could be attributed to more favorable electrostatic–misfit interactions between the gas and the nonpolar region of the fluids.


 Please wait while we load your content...
Please wait while we load your content...