Studies of the stability, nucleophilic substitution reactions, DNA/BSA interactions, cytotoxic activity, DFT and molecular docking of some tetra- and penta-coordinated gold(iii) complexes†
Abstract
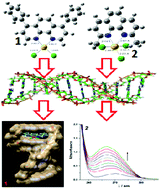
Two gold(III) complexes, square-planar [Au(DPP)Cl2]+(1) and distorted square-pyramidal [Au(DMP)Cl3] (2) (where DMP = 2,9-dimethyl-1,10-phenanthroline and DPP = 4,7-diphenyl-1,10-phenanthroline), were studied by different experimental methods. Their stability in water and in buffer solution (25 mM Hepes, 30 mM NaCl, pH = 7.2) was investigated by UV-Vis spectroscopy while their redox stability is confirmed by CV. Substitution reactions between complexes 1 and 2, and biologically relevant ligands, such as thiourea (Tu), guanosine-5′-monophosphate (5′-GMP), glutathione (GSH) and L-methionine (L-Met), were studied by a stopped-flow technique, under the pseudo-first order conditions as a function of ligand concentration and temperature. According to the values of the activation parameters, all studied reactions followed an associative substitution mechanism. DNA binding studies of complexes 1 and 2 were performed by UV-Vis and fluorescence spectroscopy and viscosity measurements, as well as interactions with bovine serum albumin (BSA). Density functional theory (DFT) was implemented in order to analyse the wave function of the optimized structures to get better insight into the binding interactions between the inert ligands and gold(III) center. The experimental results of binding studies with DNA and BSA were simulated and compared by performing a molecular docking study. All results demonstrate the strong connection between the reactivity of the complexes toward biologically important targets and their structural and electronic characteristics. The cytotoxic activity of complexes 1 and 2 against different cell lines (MDA-MB-231, HCT-116, and HaCaT) was evaluated 24 and 72 h after treatments. The results indicate reduced viability of cell lines in a time- and dose-dependent manner.


 Please wait while we load your content...
Please wait while we load your content...