Interaction of natural flavonoid eriocitrin with β-cyclodextrin and hydroxypropyl-β-cyclodextrin: an NMR and molecular dynamics investigation†
Abstract
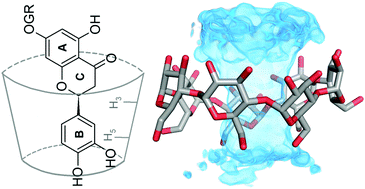
It is well known that flavanones have beneficial health effects. Eriodictyol-7-rutinoside, also called eriocitrin, is a flavanone with many positive effects. Like many of these compounds, low water solubility, stability and shelf life are major issues. Many studies have focused on improving these aspects. Towards this goal, β-cyclodextrin and its derivatives are interesting candidates. These compounds are characterized by a hydrophobic central cavity that usually enhances ligand solubility in aqueous solutions, affecting the chemical characteristics of the encapsulated ligand. Recently, eriocitrin/hydroxypropyl-β-cyclodextrin complex with advanced properties has been reported. Herein, computational approaches were combined with NMR spectroscopy to characterize the eriocitrin/β-cyclodextrin complex and compare it with that of eriocitrin/hydroxypropyl-β-cyclodextrin.


 Please wait while we load your content...
Please wait while we load your content...