Tuning the morphology and redox behaviour by varying the concentration of Fe in a CoNiFe ternary oxide heterostructure for hybrid devices
Abstract
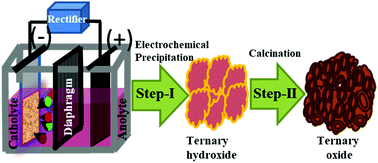
The effect of the incorporation of a third transition metal cation (Fe–O) and its concentration on the configuration of cobalt nickel oxide has been studied. An attempt has been made to synthesize hierarchical porous cobalt–nickel–iron ternary oxide heterostructure arrays by a simple two-step approach, which is a novel galvanostatic electrochemical technique, followed by calcination. The synthesized ternary oxide heterostructures have been targeted as an electrode for hybrid capacitor application due to their high redox potentials. A diaphragm cell was used for the electrodeposition of the ternary hydroxide at room temperature from a nitrate bath of the corresponding metal ions at a pH of 2. A series of experiments was carried out with a fixed concentration of Co (30 g dm−3) and Ni (30 g dm−3) while varying the concentration of Fe (10, 20 and 30 g dm−3) at the optimized pH of 2. The electrodeposited ternary metal hydroxides were calcined at an optimum temperature of 300 °C for 2 h to obtain the ternary oxide heterostructure. The formation of the ternary oxide heterostructure has been confirmed by X-ray diffraction analysis. The field emission scanning electron microscopy images suggest the formation of a hierarchical nanoflower architecture consisting of an interconnected flower petal with a porous surface and spherical nano crystallites at various Fe concentrations. Transmission electron microscopy supports the formation of nano crystallites with the size of ∼20 nm. Elemental analysis of the ternary oxides was carried out by using atomic absorption spectrophotometry, which suggests that all the materials contain Co, Ni, and Fe in various ratios. The result showed that with an increase in Fe concentration from 10 to 30 g dm−3 with the fixed concentration of Co and Ni, the % iron content in the ternary composite increases with a decrease in the concentration of Co and Ni. The increasing concentration of Fe in the metal oxide decreases the specific capacitance from 440 F g−1 to 272 F g−1, indicating a significant difference in the observed redox processes. However, the cycling stability for both these samples showed an excellent retention of over 92% after 1000 cycles.


 Please wait while we load your content...
Please wait while we load your content...