Influence of the pendant arm in deoxydehydration catalyzed by dioxomolybdenum complexes supported by amine bisphenolate ligands†
Abstract
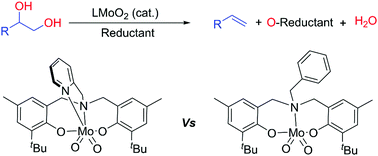
Dioxomolybdenum complexes supported by aminebisphenolate ligands were evaluated for their potential in catalyzing the deoxydehydration (DODH) reaction to establish structure–activity relationships. The nature of the pendant arm in these aminebisphenolate ligands was found to be crucial in determining reactivity in the deoxydehydration of styrene glycol (1-phenyl-1,2-ethanediol) to styrene. Pendant arms bearing strongly coordinating N-based groups such as pyridyl or amino substituents were found to hinder activity while those bearing non-coordinating pendant arms (benzyl) or even weakly coordinating groups (an ether) resulted in up to 6 fold enhancement in catalytic activity. A dioxomolybdenum complex featuring an aminemonophenolate ligand derived from the aminebisphenolate skeleton also resulted in similar yield enhancements. Although aromatic solvents were found to be ideal for performing these catalytic reactions, polar solvents such as N,N-dimethylformamide (DMF) and N,N′-dimethylpropyleneurea (DMPU) were also suitable. The catalyst was found to maintain its structural integrity under the optimized conditions and could be recycled for a second catalytic run without loss of activity. With the activated substrate meso-hydrobenzoin, trans-stilbene was obtained in a 56% yield at 220 °C along with benzaldehyde (71%) suggesting that the diol is a competing reductant under these conditions.


 Please wait while we load your content...
Please wait while we load your content...