A theoretical study on the pKa values of selenium compounds in aqueous solution
Abstract
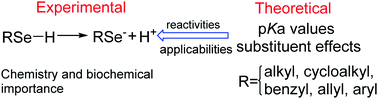
The importance of selenium compounds in chemistry and biochemistry has become apparent. The pKa values are important for understanding the reactivity and applicability of selenium compounds. To this end, we calculated the the pKa values of 16 selenols in aqueous solution by using two kinds of DFT methods and PCM-Bondi, SMD and SMDsSAS models. It is observed that the highest precision with a lowest RMSE value of 0.38 pK units was provided by using the ωB97XD method with the SMD model. Therefore, this theoretical protocol was employed to calculate the pKa values of selenol compounds and selenoxo forms of selenocarboxylic acid compounds including R = alkyl, cycloalkyl, benzyl, allyl and aryl as well as the substituent effects. The results suggest that selenols can give higher pKa values than selenocarboxylic acids. Generally, for both substituted selenols and selenocarboxylic acids, EWGs can decrease the pKa values and EDGs can increase the pKa values, and the pKa of the EWG of –COOH is larger and the pKa values of the EDGs of –NH2 and –N(CH3)2 are smaller. In addition, linear relationships between the pKa values with the natural charges of Se atoms, EHOMO, etc. were obtained in different types of selenols and selenocarboxylic acids.


 Please wait while we load your content...
Please wait while we load your content...