Toward novel sulphur-containing derivatives of tetraazacyclododecane: synthesis, acid–base properties, spectroscopic characterization, DFT calculations, and cadmium(ii) complex formation in aqueous solution†
Abstract
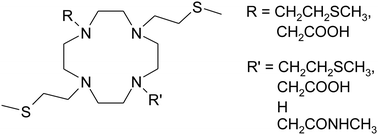
Macrocyclic ligands obtained by N-functionalization of 1,4,7,10-tetraazacyclododecane (cyclen) have been widely studied due to their remarkable complexing properties toward a variety of transition metals and lanthanides. Despite a plethora of cyclen-based molecules described in the literature, ones bearing sulphur-containing functional groups have been almost ignored. Herein, a novel series of derivatives with hanging sulphide side-arms have been investigated: 1,4,7,10-tetrakis[2-(methylsulfanyl)ethyl]-1,4,7,10-tetraazacyclododecane (DO4S), 1,4,7,tris[2-(methylsulfanyl)ethyl]-1,4,7,10-tetraazacyclododecane (DO3S), 1,4,7,tris[2-(methylsulfanyl)ethyl]-10-methylacetamido-1,4,7,10-tetraazacyclododecane (DO3SAm), and 1,7,bis[2-(methylsulfanyl)ethyl]-4,10-diacetic-1,4,7,10-tetraazacyclododecane (DO2A2S). 1,4,7,10-Tetra-n-buthyl-1,4,7,10-tetraazacyclododecane (DOT-n-Bu) was included as well in this study for comparison purposes. These compounds have been synthesized and then experimentally and theoretically characterized. Their protonation constants (pKa) have been determined at 25 °C in 0.15 M aqueous NaNO3 and in 0.15 M aqueous tetramethylammonium chloride by potentiometric titrations and partly by UV-vis spectrophotometric measurements. Density functional theory (DFT) calculations have been performed for cyclen, DO4S, and DO3S to investigate the conformations, the thermodynamics of protonation equilibria and to rationalize the relevant electronic transitions. Stability constants of the Na+ complexes (log βNa) were computed for DO4S, DO3S, DO3SAm, and DO2A2S. For all compounds, the monodimensional 1H-NMR and bidimensional (COSY, NOESY, and HMQC) spectra have been obtained in D2O as a function of pD. Results indicate that sulphur-containing pendant arms induce partly unpredictable pKa, NMR, UV-Vis, and log βNa properties on the molecules, and that these properties significantly differ from those of the corresponding compounds without sulphur (e.g. cyclen and DOT-n-Bu). Furthermore, potentiometric and 1H NMR titrations were performed in order to evaluate the complexation ability of DO4S, DO3S and DO2A2S toward Cd2+ as a case-example of soft metal ions. The obtained complexes show remarkable stability and are stronger than those formed with cyclen and its most common derivative DOTA especially at acidic pH, thus demonstrating that these compounds can be promising chelators of soft metal ions.


 Please wait while we load your content...
Please wait while we load your content...