Dicopper(ii) tetrapyridyl complexes incorporated with ancillary ligands for effective water oxidation†
Abstract
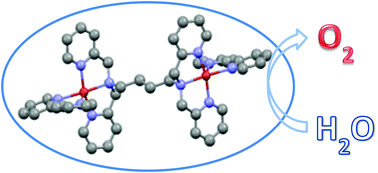
The water oxidation reaction (WOR) is a bottleneck in water splitting. Though many catalysts have been developed for this reaction, cost-effective, robust and efficient electrocatalysts based on non-precious metals are still highly desired. Herein reported are the synthesis, characterization and electrocatalytic properties of a dicopper(II) complex of the tetrapyridyl ligand N,N,N′,N′-tetrakis(2-pyridylmethyl)-1,4-butanediamine (tpbn) and 2,2′-bipyridine (bpy), [Cu2(tpbn)(bpy)2](ClO4)4·2CH3COCH3 (1). The X-ray single crystallography analysis revealed that the tpbn ligand bridges two copper(II) centers located in a square pyramidal environment composed of one bpy ligand as well as two pyridyl and one amino groups of a tpbn. Complex 1 was a molecular electrocatalyst for WOR in 0.1 M NaOAc–NaOH solutions of pH 11.0–14.0. It was demonstrated that the incorporation of the ancillary ligand bpy into the tpbn-bridged dicopper(II) complex is crucial for the stabilization of the catalytic system and the enhancement of the activity. This work provided an alternative to achieve robust homogeneous catalytic systems with better performance.


 Please wait while we load your content...
Please wait while we load your content...