Adsorption of methylene blue onto porous carbon materials prepared from Na2EDTA
Abstract
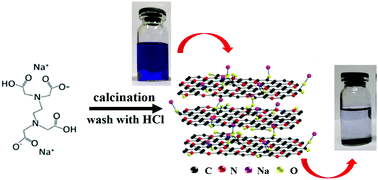
Disodium ethylenediamine tetraacetate (Na2EDTA) is a commonly used reagent in laboratory, which has high oxygen and nitrogen content. In this study, Na2EDTA was employed as a precursor to fabricate porous carbon (PC) materials through a one-step pyrolysis process at different temperatures (650 and 750 °C). The obtained PC samples were systematically characterized and employed as adsorbents for methylene blue (MB) adsorption by batch experiments. The results showed that the PC samples possessed satisfying specific surface area and pore volume through the direct one-step pyrolysis process and a higher pyrolysis temperature (750 °C) favored the formation of mesopores. The formation of the pore structure can be attributed to the existence of a large amount of alkaline functional groups in Na2EDTA, which exhibited a pore-making effect during the pyrolysis process. Adsorption experiments indicated that MB binding onto the PC samples obeyed the pseudo-second-order kinetic model and Langmuir isotherm model. The maximum adsorption capacities calculated using the Langmuir model were 734.4 and 1354.7 mg g−1 for PC650 and PC750, respectively, which are comparable to or much higher than those of most of the adsorbents reported recently. Furthermore, the PC sample exhibited extensive adsorption performance for other dyes including cation and anion dyes. In addition, the carbon materials can be regenerated with ethanol and still maintained high adsorption performance even after 4 regeneration cycles, indicating superior recyclability. This study provided a simple one-step pyrolysis method to prepare porous carbon materials through self-activation effects.


 Please wait while we load your content...
Please wait while we load your content...