Synthesis and anti-proliferative activities of amine capped Pd and Pt macrocycles of 4,4′-dipyridylselenides†
Abstract
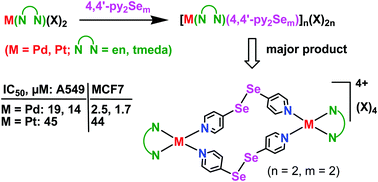
The synthesis, characterization and anti-proliferative properties of products formed from the reactions of amine-capped PdII and PtII complexes and 4,4′-dipyridylselenide/diselenide have been investigated. The addition of cis-M(N∩N)(X)2 with 4,4′-py2Sem yielded stable macrocyclic complexes [M(N∩N)(py2Sem)]n(X)2n (M = Pd, Pt; N∩N = en, tmeda; m = 1 or 2; X = NO3, OTf). The 77Se, 195Pt NMR and ESI mass spectra of the product of the diselenide ligand showed the presence of two types of complexes which are indistinguishable by 1H NMR spectroscopy. Their relative ratio depends on the nature of N∩N and M. The major product has been confirmed as a macrocyclic dimeric complex [Pd(tmeda)(μ2-4,4′-py2Se2)]2(OTf)4 containing a “Pd–py–Se–Se–py–Pd” linkage by single crystal XRD. In vitro evaluation of the water-soluble complexes using lung and breast carcinoma cells (A549 and MCF7) showed high anti-proliferative activity of the Pd macrocycles (IC50 = 1.7–19 μM).


 Please wait while we load your content...
Please wait while we load your content...