Biological promiscuity of a binuclear Cu(ii) complex of aminoguanidine Schiff base: DNA binding, anticancer activity and histidine sensing ability of the complex†
Abstract
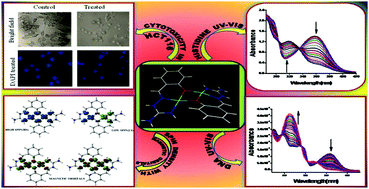
A tridentate N,N,O-donor ligand, in the form of a Schiff base of aminoguanidine (LH) with salicylaldehyde, and its Cu(II) complex, [Cu2(LH)2](ClO4)2, have been synthesized. The single crystal X-ray structure of the complex is reported. The complex shows strong DNA binding affinity, with a binding constant value comparable to that of classical intercalators like ethidium bromide. Against the human colon cancer cell line HCT116, the complex shows significant in vitro cytotoxicity with a LD50 value of 20 μM. It is shown that the cytotoxicity of the complex follows the apoptosis mechanism. The complex can also be used for selective spectrophotometric or fluorometric determination of histidine. The binuclear Cu(II) complex shows strong intramolecular antiferromagnetic interaction between the Cu(II) atoms mediated by two bridging phenoxide ligands, with 2J = −656 cm−1. DFT calculations are used to explain the magnetic interaction.


 Please wait while we load your content...
Please wait while we load your content...