Slow magnetic relaxation in O–Se–O bridged manganese(iii) Schiff base complexes†
Abstract
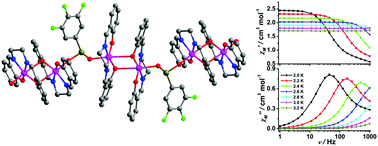
Two new chain complexes consisting of a Mn(salen) building block bridged by O–Se–O units, [Mn2(salen)2(L)](ClO4) (1) and {[Mn(salen)]2(L)2}·Y (2) (salen = N,N′-bis(salicylidene)-ethylenediamine, L = 3,4,5-trifluorobenzeneseleninic acid, Y = salicylaldehyde) have been synthesized and characterized structurally and magnetically. In the two complexes above, the 3,4,5-trifluorobenzeneseleninic acid ligand adopts a syn–anti bridging coordination mode and all the MnIII ions show a distorted octahedral geometry. In complex 1, the [Mn2(salen)2]2+ dimers are connected alternatively by the bridging ligands into an infinite zigzag chain, while the [Mn(salen)]+ monomers are connected alternatively in complex 2. The magnetic measurements reveal that both complexes exhibit slow magnetic relaxation behaviours with an effective energy barrier Ueff = 31.2 K for 1 and Ueff = 35.1 K for 2, respectively. These compounds are rare examples of a small number of aromatic selenite based magnetic materials.


 Please wait while we load your content...
Please wait while we load your content...