The promotion of proton conductivity by immobilizing molybdovanadophosphoric acids in metal–organic frameworks†
Abstract
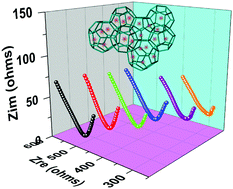
The expansion of metal–organic frameworks (MOFs) into new proton-conducting platforms has aroused special interest due to their defined frameworks coupled with chemically adjustable porosities. Immobilizing polyoxometalates (POMs) into MOFs can adequately intensify the superiorities of the POM guest and MOF matrix in the proton conducting field; however, vanadium-substituted POMs as guests in MOFs for conductivity have attracted limited attention compared to the parent Keggin polyanions. Herein, a series of molybdovanadophosphoric acids (denoted PMoVx) are impregnated into the MIL-101 framework to obtain PMoVx@MIL-101 composites for proton conduction. The effects of proton number and POM loading amounts on the conductive behaviour of POM@MOF composites have been documented. Additionally, the composites are incorporated into polyvinyl alcohol/polyvinyl pyrrolidone (PVA/PVP) blends to fabricate hybrid membranes. Preferable proton conductivities are achieved with PMoV2@MIL-101-11.2 (6.31 × 10−3 S cm−1) along with its hybrid membrane (1.96 × 10−3 S cm−1) at ∼98% RH and 353 K; these values are about two orders of magnitude higher than those of the parent MIL-101. This work contributes to driving further advances of polyoxometalate-based MOF composites in similar electrochemical apparatus.


 Please wait while we load your content...
Please wait while we load your content...