Synthesis of blue emissive functionalized 9,9-disubstituted fluorene derivatives via BF3·OEt2 mediated reaction of co-planar 9-(phenylethynyl)-9H-fluoren-9-ols with isatin imines†
Abstract
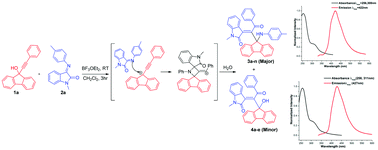
A Lewis acid mediated reaction of 9-(phenylethynyl)-9H-fluoren-9-ols with various isatin imines afforded a number of blue emissive 3-(2-oxo-2-phenyl-1-(9-(phenylamino)-9H-fluoren-9-yl)ethylidene)indolin-2-one and 3-(1-(9-hydroxy-9H-fluoren-9-yl)-2-oxo-2-phenylethylidene)indolin-2-one derivatives in excellent combined yield. A plausible mechanism for the formation of title compounds involving two pathways is explained via incorporation of a propargyl cation and a common fluorene-9-spiroazitidine intermediate is proposed. The structure of representative compounds was established by the single crystal XRD method. Evaluation of photophysical properties such as absorption, solvatochromism, emission, Stokes shift and quantum yields revealed that the compounds are blue emissive in nature.


 Please wait while we load your content...
Please wait while we load your content...