Dipolar cycloadditions of HMF-based nitrones: stepwise and multicomponent reactions, stereochemical outcome and structural scope†
Abstract
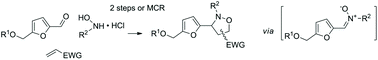
The straightforward preparation of new 3-furanyl isoxazolidines by 1,3-dipolar cycloaddition of 5-hydroxymetylfurfural (HMF)-derived nitrones with electro-deficient olefins is investigated. The study addresses the stepwise reaction, with isolation of the intermediate nitrone, and the multicomponent protocol involving directly HMF and other reaction partners and reagents. The optimal reaction parameters (solvent, temperature, stoichiometry) for the stepwise and multicomponent reactions are defined, resulting in a choice of clean and direct conditions leading to cycloadducts in good yields. Isopropanol is found to be the most appropriate solvent in terms of both acceptability and efficiency depending on the solubility of the different substrates and on specific requirements for the stepwise and multicomponent protocols. The stereochemical outcome of the reaction and the structural scope with respect to various dipolarophiles (acrylates, cycloalkenones, acrylamides, acrylonitrile) and variously substituted hydroxylamines, using HMF and O-substituted HMF analogs are also fully assessed. The structural considerations allowing more regioselective reactions are also defined. This study provides the basic elements necessary for considering the use of HMF in such strategies.


 Please wait while we load your content...
Please wait while we load your content...