Thiol-promoted catalytic synthesis of high-performance furan-containing lubricant base oils from biomass derived 2-alkylfurans and ketones†
Abstract
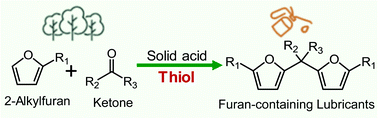
About 97% of lubricant base oils are currently sourced from petroleum and the majority of the rest is produced from vegetable oils. We demonstrate a promising catalytic route to produce base oils from lignocellulosic biomass-derived 2-alkylfurans and ketones via carbon–carbon coupling in neat conditions. Among several homogeneous and heterogeneous acid catalysts tested, a perfluorinated sulfonic acid (Aquivion PW79S) exhibits the best catalytic performance and yields up to 90% renewable furan-containing base oils with the use of a thiol promotor. The effects of the acid strength of the catalysts, molecular size and structure of thiols and ketones, and fraction of thiols are studied. Electronic structure calculations elucidate the reaction pathway and indicate that the thiol reduces the barrier of the rate-determining dehydration step. The structure and properties of base oils can be tuned by using different synthons. These base oils have excellent properties and can be competitive with or surpass the commercial synthetic alkylnaphthalene and furan-containing bio-ester base oils.


 Please wait while we load your content...
Please wait while we load your content...