In situ electrosynthesis of anthraquinone electrolytes in aqueous flow batteries†
Abstract
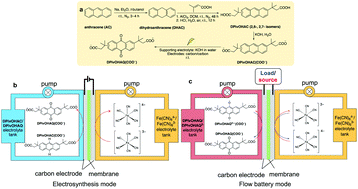
We demonstrate the electrochemical oxidation of an anthracene derivative to a redox-active anthraquinone at room temperature in a flow cell without the use of hazardous oxidants or noble metal catalysts. The anthraquinone, generated in situ, was used as the active species in a flow battery electrolyte without further modification or purification. This potentially scalable, safe, green, and economical electrosynthetic method is also applied to another anthracene-based derivative and may be extended to other redox-active aromatics.
- This article is part of the themed collection: 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...