Construction of noble-metal alloys of cobalt confined N-doped carbon polyhedra toward efficient water splitting†
Abstract
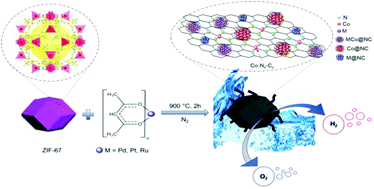
The quest for an efficient electrocatalyst for a water splitting reaction to produce hydrogen has driven researchers to develop new eco-friendly catalysts. Herein, we report a universal avenue to synthesize alloys of Co encapsulated within nitrogen-doped carbon (NC) polyhedra along with short carbon nanotubes (CNTs) derived from the metal–organic framework (MOF). The reported synthesis stands out being an environmentally benign way to synthesize such hybrids in situ since it is a one-step, hydrogen-free method and uses a single source precursor that successfully overcomes the hurdles of traditional synthesis methods. The as-synthesized MCo@NC (M = Pt, Pd, Ru) shows bifunctional catalytic activity competing with the state-of-the-art catalyst Pt/C (20 wt%) and RuO2 towards the hydrogen evolution and oxygen evolution reactions (HER and OER), respectively, in alkaline media. The best HER activity is observed for PtCo@NC (E@10 mA cm−2 = 38 mV), whereas the best OER activity is observed for RuCo@NC (E@10 mA cm−2 = 280 mV). A total water splitting electrolyzer set up with PtCo@NC||RuCo@NC (cathode||anode) showed an impressive 1.52 V of onset potential. A synergistic effect between the bimetallic MCo@NC moiety, Co–Nx centers, and Co nanoparticles wrapped in N-doped graphitic layers (Co@NC) is believed to be the cause for enhanced catalytic activity.
- This article is part of the themed collection: #RSCPoster Conference


 Please wait while we load your content...
Please wait while we load your content...