Electrochemical N-demethylation of tropane alkaloids†
Abstract
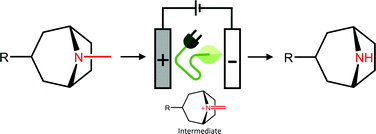
A practical, efficient, and selective electrochemical N-demethylation method of tropane alkaloids to their nortropane derivatives is described. Nortropanes, such as noratropine and norscopolamine, are important intermediates for the semi-synthesis of the medicines ipratropium or oxitropium bromide, respectively. Synthesis was performed in a simple home-made electrochemical batch cell using a porous glassy carbon electrode. The reaction proceeds at room temperature in one step in a mixture of ethanol or methanol and water. The method avoids hazardous oxidizing agents such as H2O2 or m-chloroperbenzoic acid (m-CPBA), toxic solvents such as chloroform, as well as metal-based catalysts. Various key parameters were investigated in electrochemical batch or flow cells, and the optimized conditions were used in batch and flow-cells at gram scale to synthesize noratropine in high yield and purity using a convenient liquid–liquid extraction method without any need for chromatographic purification. Mechanistic studies showed that the electrochemical N-demethylation proceeds by the formation of an iminium intermediate which is converted by water as the nucleophile. The optimized method was further applied to scopolamine, cocaine, benzatropine, homatropine and tropacocaine, showing that this is a generic way of N-demethylating tropane alkaloids to synthesize valuable precursors for pharmaceutical products.


 Please wait while we load your content...
Please wait while we load your content...